Amoxicillin*-clavulanic acid (2/1) 0.016 - 256*
Product Details:
- Ph Level Approximately 4-6 (1% solution in water)
- Poisonous No
- Application Pharmaceuticals, Veterinary medicines
- Physical Form Solid
- Shape Crystalline
- Melting Point 194-196C
- HS Code 29411030
- Click to View more
Amoxicillin*-clavulanic acid (2/1) 0.016 - 256* Price And Quantity
- 4000 INR
Amoxicillin*-clavulanic acid (2/1) 0.016 - 256* Product Specifications
- Solid
- No
- Pharmaceuticals, Veterinary medicines
- Approximately 4-6 (1% solution in water)
- 26787-78-0 (Amoxicillin), 61177-45-5 (Clavulanic Acid)
- 194-196C
- 29411030
- 200-093-0 (Amoxicillin), 262-087-5 (Clavulanic Acid)
- Crystalline
- Bitter
- Co-amoxiclav
- Powder
- 365.4 g/mol (Amoxicillin), 199.16 g/mol (Clavulanic Acid)
- Store in cool, dry place, below 25C
- Amoxicillin and Clavulanic Acid (2:1 Ratio)
- Slight Odor
- Pharmaceutical Grade
- Beta-Lactam Antibiotic
- Antibiotic Combination
- Gram per cubic centimeter(g/cm3)
- 99%
- Available on request
- Not Applicable (Decomposes)
- Treatment of bacterial infections
- White to Off-White
- 2 years
- C16H19N3O5S (Amoxicillin), C8H9NO5 (Clavulanic Acid)
Product Description
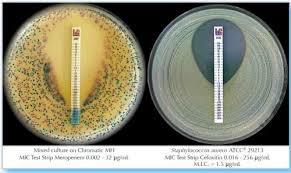
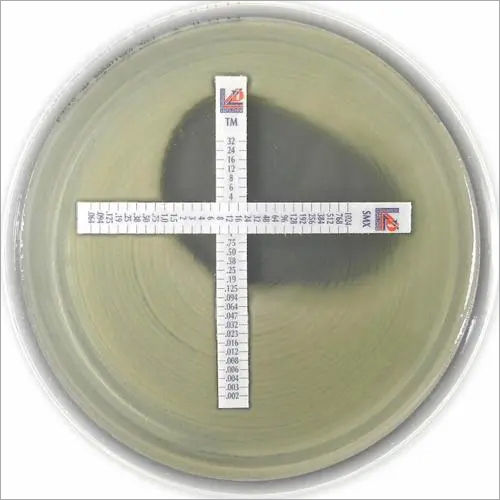
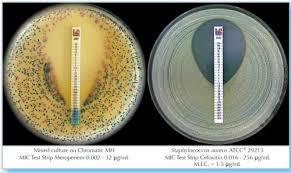
MIC Test Strip | |||||||||||||||||||
Paper strips* for determining the Minimum Inhibitory Concentration (M.I.C.) | |||||||||||||||||||
The Liofilchem MIC Test Strips is a quantitative assay for determining the Minimum Inhibitory Concentration (MIC) of antimicrobial agents against microorganisms to indicate appropriate patient treatment and for identifying resistance patterns. Liofilchem MIC Test Strips are made of special high quality paper impregnated with a predefined concentration gradient of antibiotic, across 15 two-fold dilutions like those of a conventional MIC method. | |||||||||||||||||||
On one side of the strip a MIC scale in g/mL is displayed and a code that identifies the antimicrobial agent. Liofilchem MIC Test Strips are available in a large variety of antibiotic configurations. Each configuration is available in blister packs of 10 strips, 30 strips and 100 strips. | |||||||||||||||||||
METHOD PRINCIPLE When the Liofilchem MIC Test Strip is applied onto an inoculated agar surface, the preformed exponential gradient of antimicrobial agent is transferred into the agar matrix. After 18 hours incubation or longer, a symmetrical inhibition ellipse centered along the strip is formed. The MIC is read directly from the scale in terms of g/mL, at the point where the edge of the inhibition ellipse intersects with the MIC Test Strip.
| |||||||||||||||||||
Broad-Spectrum Infection Control
Amoxicillin-clavulanic acid (2/1) is highly effective against a wide range of bacterial infections. The unique combination enhances the antibiotics effectiveness, making it valuable for clinical and veterinary applications where resistance is a concern.
Quality and Stability Assurance
Manufactured to meet stringent USP, BP, and EP standards, this product demonstrates excellent stability and purity. It maintains full efficacy for up to two years when stored between 2-8C in sealed containers, ensuring consistent pharmaceutical quality.
Flexible Packaging and Application
Available in bulk or customized packaging, the powder form allows easy integration into diverse pharmaceutical formulations and veterinary preparations. Its solubility in water ensures convenient reconstitution and precise dosing for end-use.
FAQs of Amoxicillin*-clavulanic acid (2/1) 0.016 - 256*:
Q: How should Amoxicillin-clavulanic acid (2/1) be stored to maintain stability?
A: Amoxicillin-clavulanic acid (2/1) should be stored in a cool, dry place below 25C, inside sealed containers to ensure stability and preserve its two-year shelf life.Q: What is the recommended process for preparing solutions with this antibiotic powder?
A: To prepare solutions, dissolve the measured amount of powder into water, ensuring the final concentration falls within the 0.016-256 mcg/ml range for both amoxicillin and clavulanic acid. Avoid contact with skin and inhalation during preparation.Q: Where is this product typically distributed and used?
A: This antibiotic combination is widely distributed to pharmaceutical and veterinary medicine manufacturers, importers, suppliers, and traders, particularly across Italy and other international markets.Q: When should Amoxicillin-clavulanic acid (2/1) be used in treatment?
A: It should be used when bacterial infections are suspected or confirmed, especially in cases where resistance to standard amoxicillin is a concern. Always follow professional guidance regarding dosage and duration.Q: What benefits does the combination of amoxicillin and clavulanic acid provide?
A: The combination extends antimicrobial coverage by inhibiting beta-lactamases, enzymes that render many bacteria resistant to amoxicillin, thus broadening treatment options for physicians and veterinarians.Q: Is there any particular incompatibility to consider with this product?
A: Yes, Amoxicillin-clavulanic acid (2/1) should not be mixed with strong oxidizing agents as they may degrade the active compounds.Q: What precautions should be taken when handling this powder?
A: Precautions include avoiding inhalation or direct contact with skin, using appropriate protective equipment, and working in a well-ventilated area during handling or preparation.
Price:
- 50
- 100
- 200
- 250
- 500
- 1000+
Other Products in 'MIC TEST MICRO BIOLOGY ' category
 |
SR GROUP
All Rights Reserved.(Terms of Use) Developed and Managed by Infocom Network Private Limited. |