Azithromycin 0.016 256
4000 INR
Product Details:
- Molecular Weight 749.0 g/mol
- Appearance Powder
- Shape Crystalline
- Storage Store in a cool, dry place, protected from light
- Other Names Azithromycin Dihydrate
- Shelf Life 3 Years
- HS Code 29415090
- Click to View more
X
Azithromycin 0.016 256 Price And Quantity
- 25000.00 - 25000.00 INR
- 4000 INR
Azithromycin 0.016 256 Product Specifications
- C38H72N2O12
- Azithromycin
- 617-500-5
- 113-115C
- 83905-01-5
- No
- Solid
- White to Off-White
- Antibiotic, pharmaceutical formulation
- 1.15 Gram per cubic centimeter(g/cm3)
- Bitter
- Used in treatment of bacterial infections
- Odorless
- 6.0-8.0 (10mg/ml) in water
- Available on request
- 29415090
- 99%
- Pharmaceutical Grade
- Azithromycin Dihydrate
- 3 Years
- Crystalline
- Store in a cool, dry place, protected from light
- Macrolide Antibiotic
- 749.0 g/mol
- Powder
- Pharmaceutical Raw Material
Product Description
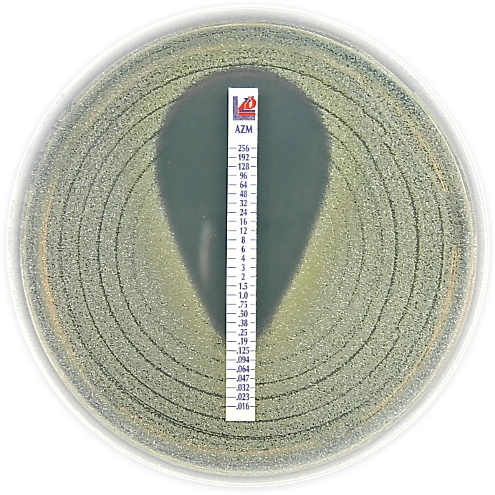
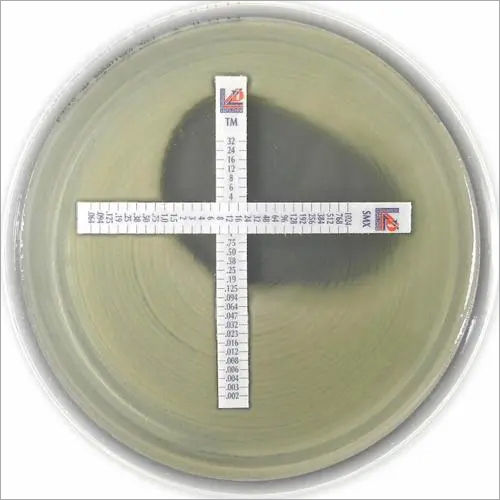
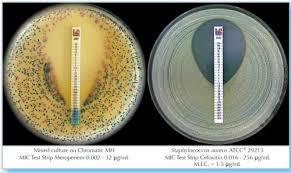
MIC Test Strip | |||||||||||||
Paper strips* for determining the Minimum Inhibitory Concentration (M.I.C.) | |||||||||||||
The Liofilchem MIC Test Strips is a quantitative assay for determining the Minimum Inhibitory Concentration (MIC) of antimicrobial agents against microorganisms to indicate appropriate patient treatment and for identifying resistance patterns. Liofilchem MIC Test Strips are made of special high quality paper impregnated with a predefined concentration gradient of antibiotic, across 15 two-fold dilutions like those of a conventional MIC method. | |||||||||||||
On one side of the strip a MIC scale in g/mL is displayed and a code that identifies the antimicrobial agent. Liofilchem MIC Test Strips are available in a large variety of antibiotic configurations. Each configuration is available in blister packs of 10 strips, 30 strips and 100 strips. | |||||||||||||
METHOD PRINCIPLE When the Liofilchem MIC Test Strip is applied onto an inoculated agar surface, the preformed exponential gradient of antimicrobial agent is transferred into the agar matrix. After 18 hours incubation or longer, a symmetrical inhibition ellipse centered along the strip is formed. The MIC is read directly from the scale in terms of g/mL, at the point where the edge of the inhibition ellipse intersects with the MIC Test Strip.
| |||||||||||||
High-Quality Raw Material for Pharmaceutical Applications
Azithromycin from this range is tailored for pharmaceutical manufacturing, presenting optimal characteristics such as high purity, stability, and standardized particle size. Its slightly soluble nature in water ensures controlled dissolution, while solubility in methanol and ethanol aids in various formulation processes. Adhering to global standards, it guarantees reliability and safety in end-products.
Safe Handling and Storage Recommendations
This product is not classified as hazardous for transportation and requires standard safety procedures. For maximum stability and shelf life, it should be stored in a cool, dry environment away from direct light. Under these conditions, azithromycin maintains a shelf life of up to three years, making it a dependable choice for continuous pharmaceutical production.
Applications and Benefits in Healthcare
Azithromycin is widely used in the formulation of medications designed to combat a range of bacterial infections. Its high assay and compliance with pharmacopeial requirements strengthen its therapeutic effectiveness. As a macrolide antibiotic, it offers a well-established benefit for healthcare providers seeking reliable solutions for treating infectious diseases.
FAQs of Azithromycin 0.016 256:
Q: How should Azithromycin 0.016 256 be stored to preserve its stability?
A: Azithromycin should be kept in a cool, dry environment and protected from light to maintain its stability. Following these storage conditions ensures its shelf life of up to three years.Q: What are the recommended applications for this Azithromycin raw material?
A: This product is primarily intended for pharmaceutical formulations as a macrolide antibiotic, used in the treatment of a range of bacterial infections.Q: When is the best time to use this product in pharmaceutical processes?
A: Azithromycin should be incorporated during the formulation phase, ensuring it is handled according to pharmacopeial guidelines for optimal quality in finished medicinal products.Q: Where does this Azithromycin meet regulatory and quality standards?
A: It complies with both USP and EP pharmacopeial standards, fulfilling requirements for impurities, microbial limits, and overall quality. These standards are internationally recognized for pharmaceutical manufacturing.Q: What is the process for ensuring the assay and purity of this product?
A: Each batch undergoes rigorous testing to guarantee a minimum assay of 98.0% on an anhydrous basis and a purity of 99%. Testing also ensures compliance with specified impurity and microbial limits.Q: How is this Azithromycin typically transported and packaged?
A: The product is shipped in 25 kg drums and is not regulated as hazardous, making transportation straightforward and secure for distributors and suppliers.Q: What are the key benefits of choosing this Azithromycin for pharmaceutical supply?
A: Its high assay, consistent quality, and compliance with international standards provide pharmaceutical manufacturers with dependable material for producing effective and safe antibiotics.Tell us about your requirement

Price:
Quantity
Select Unit
- 50
- 100
- 200
- 250
- 500
- 1000+
Additional detail
Mobile number
Email
Other Products in 'MIC TEST MICRO BIOLOGY ' category
 |
SR GROUP
All Rights Reserved.(Terms of Use) Developed and Managed by Infocom Network Private Limited. |