Ampicillin 0.016 256
4000 INR
Product Details:
- Storage Store at 28C, protect from light and moisture
- Shelf Life 3 years under recommended storage
- Taste Slightly bitter
- Ph Level 4.06.0 (1% solution)
- Grade Analytical grade
- Appearance Powder
- Boiling point Not applicable (decomposes)
- Click to View more
X
Ampicillin 0.016 256 Price And Quantity
- 4000 INR
- 100 STRIPS
- 4000.00 - 25000.00 INR
Ampicillin 0.016 256 Product Specifications
- Crystalline
- 98%
- 29411030
- Ampicillin sodium salt, D-(-)--Aminobenzylpenicillin
- 69-53-4
- Beta-lactam antibiotic
- Powder
- Not applicable (decomposes)
- 3 years under recommended storage
- 4.06.0 (1% solution)
- Ampicillin
- Slightly bitter
- Analytical grade
- Antibiotic Reference Standard
- Store at 28C, protect from light and moisture
- 200-709-7
- (Refer to product documentation)
- Microbiological testing, antibiotic research, quality control
- White to off-white
- >200C (decomposes)
- 349.4 g/mol
- C16H19N3O4S
- Solid
- Non-poisonous
- Odorless
- Used as a reference standard for antimicrobial susceptibility testing
Product Description
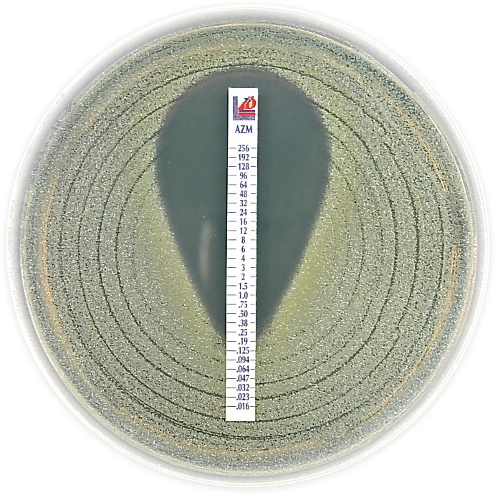
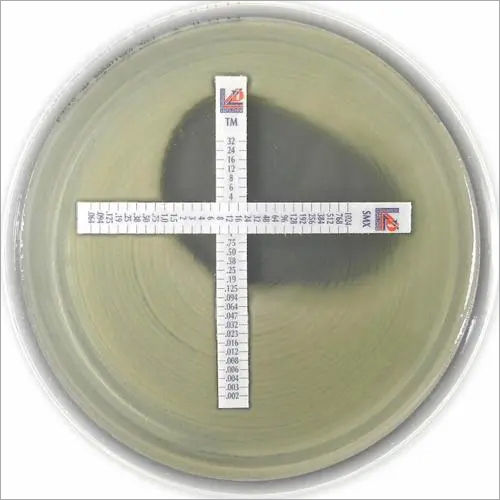
MIC Test Strip | ||||||||||
Paper strips* for determining the Minimum Inhibitory Concentration (M.I.C.) | ||||||||||
The Liofilchem MIC Test Strips is a quantitative assay for determining the Minimum Inhibitory Concentration (MIC) of antimicrobial agents against microorganisms to indicate appropriate patient treatment and for identifying resistance patterns. Liofilchem MIC Test Strips are made of special high quality paper impregnated with a predefined concentration gradient of antibiotic, across 15 two-fold dilutions like those of a conventional MIC method. | ||||||||||
On one side of the strip a MIC scale in g/mL is displayed and a code that identifies the antimicrobial agent. Liofilchem MIC Test Strips are available in a large variety of antibiotic configurations. Each configuration is available in blister packs of 10 strips, 30 strips and 100 strips. | ||||||||||
METHOD PRINCIPLE When the Liofilchem MIC Test Strip is applied onto an inoculated agar surface, the preformed exponential gradient of antimicrobial agent is transferred into the agar matrix. After 18 hours incubation or longer, a symmetrical inhibition ellipse centered along the strip is formed. The MIC is read directly from the scale in terms of g/mL, at the point where the edge of the inhibition ellipse intersects with the MIC Test Strip.
| ||||||||||
Reliable Reference Standard for Laboratory Applications
Ampicillin 0.016 256 serves as a trusted reference standard in microbiological testing and antibiotic research. Its consistent quality and precise formulation allow accurate antimicrobial susceptibility assessments, making it a valuable tool for ensuring the reliability of laboratory results. The product is tailored for ease of reconstitution and handling, offering dependable performance across diverse testing protocols.
Safe Handling and Convenient Storage
This product is classified as non-hazardous according to GHS standards and is safe to handle, non-poisonous, and odorless. It remains stable for three years when stored between 28C, protected from light and moisture. Its crystalline, solid form ensures easy preparation and minimal risk during laboratory procedures.
Compliant with International Standards
Ampicillin 0.016 256 meets rigorous international laboratory guidelines for antibiotic reference standards. Manufactured to analytical grade quality, it is suitable for use in accredited laboratories and research institutions, providing confidence in your results and compliance with regulatory requirements.
FAQs of Ampicillin 0.016 256:
Q: How should Ampicillin 0.016 256 be prepared for laboratory use?
A: Reconstitute the lyophilized powder using sterile water, as Ampicillin 0.016 256 is highly soluble in water. Ensure accurate dilution to achieve the desired reference concentration for microbiological or antibiotic susceptibility tests.Q: What is the primary application of Ampicillin 0.016 256?
A: Its main use is as a reference standard in antimicrobial susceptibility testing, microbiological research, and laboratory quality control protocols, ensuring consistent and reliable results.Q: When should this reference standard be used during testing?
A: Ampicillin 0.016 256 should be incorporated during the quality control phase of microbiological and antibiotic susceptibility assays, to calibrate processes and validate results.Q: Where can Ampicillin 0.016 256 be stored to maintain its stability?
A: Store the vial at temperatures between 28C, protected from direct light and moisture, to ensure stability and efficacy for the full three-year shelf life.Q: What are the safety and transport considerations for this product?
A: Ampicillin 0.016 256 is non-hazardous and non-poisonous, classified safe for routine laboratory handling. It can be transported under ambient conditions without special precautions, as per GHS guidelines.Q: What benefits does using this analytical grade standard offer laboratories?
A: Using this standard improves accuracy in antimicrobial assays, ensures compliance with international laboratory standards, and supports robust quality control, enhancing overall testing reliability.Tell us about your requirement

Price:
Quantity
Select Unit
- 50
- 100
- 200
- 250
- 500
- 1000+
Additional detail
Mobile number
Email
Other Products in 'MIC TEST MICRO BIOLOGY ' category
 |
SR GROUP
All Rights Reserved.(Terms of Use) Developed and Managed by Infocom Network Private Limited. |