MIC TEST STRIPS Amikacin 0.016 256 (FDA CLEARED)
Product Details:
- Usage Clinical microbiology laboratory, antimicrobial susceptibility testing
- Other Names Etest Amikacin; Amikacin MIC Test
- Shelf Life As per manufacturer, generally 3 years from date of production
- Shape Flat rectangular strip
- Structural Formula Not specified on product sheet
- Poisonous Not for human consumption; laboratory diagnostic use only
- Molecular Weight 585.6 g/mol (Amikacin Dihydrate)
- Click to View more
MIC TEST STRIPS Amikacin 0.016 256 (FDA CLEARED) Price And Quantity
- 4000.00 - 25000.00 INR
MIC TEST STRIPS Amikacin 0.016 256 (FDA CLEARED) Product Specifications
- 3822.90.90
- Determination of Minimum Inhibitory Concentration (MIC) of Amikacin
- Diagnostic/Laboratory use, FDA Cleared
- Not applicable (test strip form)
- Antibiotic (Aminoglycoside) MIC Test Strip
- 253-497-0
- Test-specific product; not applicable
- C22H43N5O132H2O (for Amikacin Dihydrate)
- Solid plastic strip
- Odorless
- Antimicrobial Susceptibility Test Strips
- White with black-printed gradient scale
- 37517-28-5
- 585.6 g/mol (Amikacin Dihydrate)
- Not for human consumption; laboratory diagnostic use only
- Plastic strip with antimicrobial gradient
- 2-8C (store refrigerated), protect from direct light
- Amikacin Dihydrate (impregnated on strip)
- Etest Amikacin; Amikacin MIC Test
- Clinical microbiology laboratory, antimicrobial susceptibility testing
- Not specified on product sheet
- Flat rectangular strip
- As per manufacturer, generally 3 years from date of production
Product Description
ANTIBIOTIC SUCCEPTIBILITY TESTING MIC Test Strip (FDA CLEARED)
| ||||||||||||||
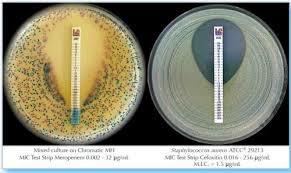
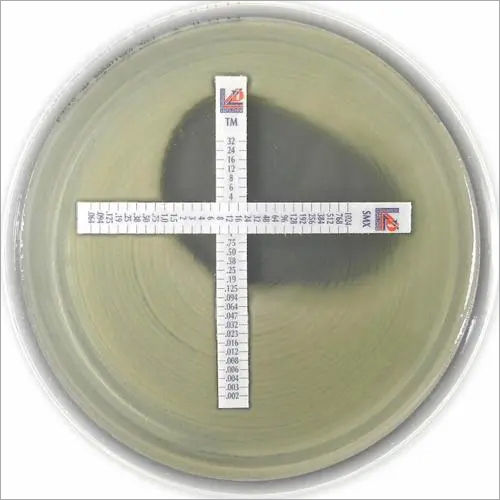
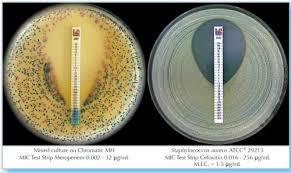
Paper strips* for determining the Minimum Inhibitory Concentration (M.I.C.)
On one side of the strip a MIC scale in g/mL is displayed and a code that identifies the antimicrobial agent. Liofilchem MIC Test Strips are available in a large variety of antibiotic configurations. Each configuration is available in blister packs of 10 strips, 30 strips and 100 strips. METHOD PRINCIPLE When the Liofilchem MIC Test Strip is applied onto an inoculated agar surface, the preformed exponential gradient of antimicrobial agent is transferred into the agar matrix. After 18 hours incubation or longer, a symmetrical inhibition ellipse centered along the strip is formed. The MIC is read directly from the scale in terms of g/mL, at the point where the edge of the inhibition ellipse intersects with the MIC Test Strip. | ||||||||||||||
Reliable Susceptibility Testing
MIC TEST STRIPS Amikacin provide accurate determination of resistance or susceptibility to Amikacin in both Gram-negative and Gram-positive non-fastidious organisms. With a precise concentration range (0.016256 g/mL) and a simple, direct visual readout, they support clear, quantitative results in clinical microbiology laboratories for effective patient care decisions.
Simple Application and Clear Results
Each sterile plastic strip is printed with an Amikacin concentration gradient. After application to inoculated agar, results can be visually interpreted at the intersection of the inhibition ellipse. This direct approach ensures efficiency and ease of use, minimizing errors and facilitating robust quality control when following the manufacturers provided instructions.
Safety and Storage
The strips are for in vitro diagnostic use only and must be handled as biohazards, with disposal managed per laboratory safety protocols. For best performance and longevity, store the strips refrigerated (28C) and protect them from direct light. Each strip is odorless and non-poisonous when used strictly as intended in laboratory diagnostics.
FAQs of MIC TEST STRIPS Amikacin 0.016 256 (FDA CLEARED):
Q: How are MIC TEST STRIPS Amikacin used in the laboratory?
A: To use, apply an individually packaged sterile strip onto the surface of an inoculated agar plate (such as Mueller-Hinton agar). Following incubation, read the MIC value where the ellipse of bacterial growth inhibition intersects the printed gradient on the strip, according to the manufacturers instructions.Q: What types of bacteria can be tested with these strips?
A: These strips are designed for the determination of Amikacin MIC against non-fastidious Gram-negative and Gram-positive organisms. They are suitable for a broad range of diagnostic applications in clinical microbiology laboratories.Q: When should MIC TEST STRIPS Amikacin be used during patient testing?
A: They are employed during antimicrobial susceptibility testing when clinicians require precise MIC values for Amikacin to guide effective antibiotic therapy, especially in cases involving non-fastidious bacteria that may have varied resistance levels.Q: Where should these test strips be stored to maintain performance?
A: Store MIC TEST STRIPS Amikacin refrigerated at 28C and protect them from direct light. Proper storage helps maintain strip integrity and prolongs shelf life, typically up to three years from the date of production.Q: What is the benefit of using a direct visual readout for MIC determination?
A: The direct visual readout at the inhibition ellipse intersection provides a straightforward, accurate way to determine the precise MIC value, reducing interpretation errors and improving efficiency in routine laboratory workflows.Q: How should used strips be disposed of after testing?
A: Used strips are considered biohazardous waste and must be disposed of in accordance with your laboratorys safety protocols and local biohazard disposal regulations.
Price:
- 50
- 100
- 200
- 250
- 500
- 1000+
Other Products in 'MIC TEST MICRO BIOLOGY ' category
 |
SR GROUP
All Rights Reserved.(Terms of Use) Developed and Managed by Infocom Network Private Limited. |