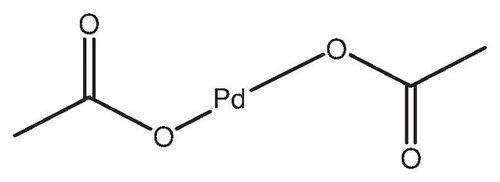
Palladium acetate
2000 INR
Product Details:
- Physical Form Solid
- Other Names Palladium diacetate; C4H6O4Pd
- Smell Odorless
- Melting Point Approx. 150C (decomposes)
- HS Code 28439090
- Molecular Weight 224.52 g/mol
- Poisonous Yes
- Click to View more
X
Palladium acetate Price And Quantity
- 1 Bottle
- 2000 INR
- 2000.00 - 6170.00 INR
Palladium acetate Product Specifications
- C4H6O4Pd
- Laboratory/Reagent Grade
- Yes
- Chemical Compound
- Palladium(II) acetate
- 224.52 g/mol
- 28439090
- Odorless
- Approx. 150C (decomposes)
- Dark red to brown
- 222-055-4
- Palladium diacetate; C4H6O4Pd
- Transition Metal Compound
- Solid
- 1.98 Gram per cubic centimeter(g/cm3)
- 99%
- Catalyst in organic synthesis
- 12 months
- Crystalline
- Store in tightly closed container, in a cool, dry, well-ventilated area away from incompatible substances
- Used in catalytic carbon-carbon and carbon-heteroatom coupling reactions
- Crystalline Powder
- Not Applicable (decomposes)
- [(CH3COO)2Pd]
- 3375-31-3
Palladium acetate Trade Information
- DELHI
- 10-15 Days
- Yes
- Contact us for information regarding our sample policy
- All India
Product Description
Palladium acetate cas number 3375-31-3
| Structure | 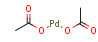 |
| Catalog No. | OS-7404 |
| Name | Palladium acetate |
| Alt. name | Palladium(II) acetate |
| CAS number | 3375-31-3 |
| Related CAS | |
| MFCD number | MFCD00012453 |
| Purity | 98% |
| Formula | C4H6O4Pd |
| FW | 224.5 |
| Note | |
| Storage | Room temperature |
| Shipping | Normal |
Versatile Catalyst for Organic Synthesis
Palladium(II) acetate is widely recognized for its catalytic properties in synthetic chemistry labs and industries. Its efficiency in facilitating carbon-carbon and carbon-heteroatom bond formation makes it a preferred choice among researchers developing pharmaceuticals, agrochemicals, and advanced materials.
Safe Handling and Storage Guidelines
To ensure safety and maintain compound stability, store Palladium(II) acetate in a tightly closed container in a cool, dry, and well-ventilated area, away from incompatible substances such as oxidizing or reducing agents. Follow recommended protocols and use proper PPE when handling this chemical.
Customizable Packaging Options
Available in 1g, 5g, 10g, 25g, and tailored packaging sizes, Palladium(II) acetate meets the diverse requirements of laboratories and industrial users. This flexibility allows customers to purchase the right amount for their specific needs, reducing waste and optimizing cost.
FAQs of Palladium acetate:
Q: How should Palladium(II) acetate be stored for optimal stability?
A: For optimal stability, Palladium(II) acetate should be kept in a tightly closed container, stored in a cool, dry, and well-ventilated area, away from incompatible substances like oxidizing or reducing agents.Q: What are the recommended applications or uses for Palladium(II) acetate?
A: Palladium(II) acetate is primarily used as a catalyst in organic synthesis, particularly in catalytic carbon-carbon and carbon-heteroatom coupling reactions for the preparation of pharmaceuticals, agrochemicals, and specialty materials.Q: When does Palladium(II) acetate decompose, and what is its melting point?
A: Palladium(II) acetate decomposes at approximately 150C instead of melting, so it should not be heated beyond this point during storage or use.Q: Where can Palladium(II) acetate be purchased and in what packaging options?
A: This compound is available through distributors, importers, suppliers, and traders in the United States, and can be purchased in packaging sizes of 1g, 5g, 10g, 25g, or in custom quantities as needed.Q: What precautions should be taken when handling Palladium(II) acetate?
A: It is important to wear appropriate protective equipment, such as gloves and safety goggles, as Palladium(II) acetate is harmful if swallowed and can cause skin irritation. Work in a well-ventilated area and avoid contact with incompatible substances.Q: How can Palladium(II) acetate benefit my synthesis process?
A: Using Palladium(II) acetate as a catalyst greatly enhances the efficiency and selectivity of organic transformations, particularly in carbon coupling reactions, resulting in higher yields and faster reaction times.Tell us about your requirement

Price:
Quantity
Select Unit
- 50
- 100
- 200
- 250
- 500
- 1000+
Additional detail
Mobile number
Email
Other Products in 'LABORATORY CHEMICAL' category
 |
SR GROUP
All Rights Reserved.(Terms of Use) Developed and Managed by Infocom Network Private Limited. |